

 |
 |
|
NNDC Databases: NuDat | NSR | XUNDL | ENSDF | MIRD | ENDF | CSISRS | Sigma |
|
|
Proton DecayThe atomic nucleus is composed of protons and neutrons. The escape of a proton is known as proton decay. Protons are bound by an attractive force from the remaining nucleons, however they also experience a repulsive force due to the Coulomb interaction with the remaining protons. Additionally, the proton angular momentum will push it towards the surface of the nucleus. In proton decay, the original nucleus with Z proton, N neutrons, and A total of nucleons will lose 1 protons and 2 nucleon: proton decay: (Z,N,A) -> (Z-1,N,A-1) The energy available for the decay is known as α- decay Q-value. For transitions between nuclear ground states it is calculated as: proton- decay Q-value=Mass(Z,N) - Mass(Z-1,N) - Mass(proton) Numerical values can be obtained from the web application QCalc. Proton decay has been observed in nuclei with a strong deficiency of neutrons, that is, with an large excess of protons. Nuclei that exhibit proton decay are indicated with a orange background in the chart of nuclei: 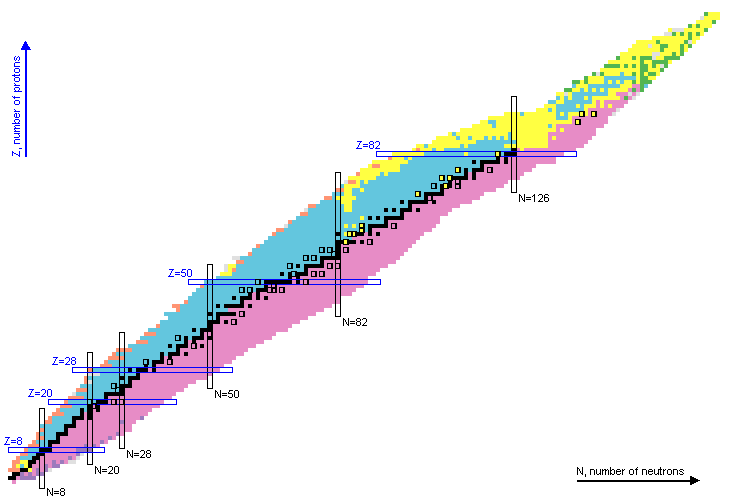 For a nucleus to exhibit proton radioactivity, the proton must have a positive value of energy inside the nucleus, which can only happen on the proton rich edge of the chart of nuclei. The proton drip-line is defined as the boundary between nuclei with negative and positive proton decay Q-value. Proton decay half-lives span from very small values up to the second range. The larger the energy available for the decay, the shorter the half-life. Additionally, nuclear levels with similar values of spin and parity to the initial level are preferentially populated. The larger the difference in angular momentum the larger the half-life. More information on proton decay can be found on this article. |